Six Things You Should Know About IVG
By Dr Zoe Bolton, published 22nd August 2024
In vitro gametogenesis (IVG), the creation of eggs and sperm from non-reproductive cells, such as skin cells, or embryonic stem cells, has the potential to revolutionise human reproduction. At a recent IVG Ethics and Policy Symposium, we brought together representatives from European bioethics commissions and committees, academics and scientists, and the CEO of a US IVG biotech start-up to discuss the urgent issues raised by this new biotechnology. Here are six key takeaways.
1. The science is rapidly advancing but there are challenges
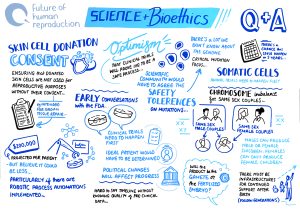
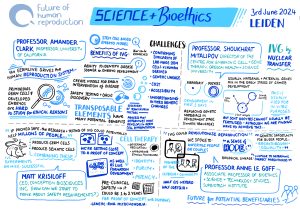
 Research into the development of in vitro derived (IVD) gametes has led to notable successes with mouse models. In 2021, the first functional IVD mouse sperm cells were reported and then, in 2023, the creation of mice with two biological fathers, marking the first time that viable IVD eggs had been generated from male cells. More recently, an alternative IVG approach to creating eggs has been developed using Somatic Cell Nuclear Transfer (a form of cloning) to create an egg by replacing the nucleus from a donor mouse egg with a nucleus taken from a mouse skin cell.
Research into the development of in vitro derived (IVD) gametes has led to notable successes with mouse models. In 2021, the first functional IVD mouse sperm cells were reported and then, in 2023, the creation of mice with two biological fathers, marking the first time that viable IVD eggs had been generated from male cells. More recently, an alternative IVG approach to creating eggs has been developed using Somatic Cell Nuclear Transfer (a form of cloning) to create an egg by replacing the nucleus from a donor mouse egg with a nucleus taken from a mouse skin cell.
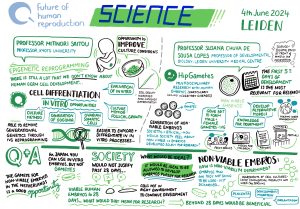
While the advances in mouse models are promising, they are not proving as easy to replicate with human germ (egg and sperm) cells. This is primarily because the mechanism of human germ cell development is still largely unknown. However, there has been a recent breakthrough in the creation of human IVD gametes – in June 2024 it was reported that human cells that were originally skin cells have been reprogrammed into early-stage sperm and egg precursors – which means that scientists are almost at the stage where IVD sperm and immature egg cells are possible.
Despite this breakthrough, there are a number of technical challenges that still need to be overcome. For example, there isn’t yet a viable culture method for developing immature human eggs into functional gametes, and creating the medium for this is a painstaking process of trial and error. There is also an issue with the high number of genetic mutations that occur when donor cells are being reprogrammed in an attempt to create germ cells. Such genetic mutations will always occur in cell transformation but a consensus will need to be reached about what the tolerance is for the number of genetic errors and whether this will ever be at a limit considered safe.
There are differing opinions within the IVG research community about when, and indeed whether, fully viable human IVD gametes will be realised in the laboratory. Optimistic forecasts suggest that the first proof of concept egg could be available within two years, while more conservative forecasts suggest ten years. There are also those who remain sceptical about whether the creation of human IVD gametes will ever be achieved. Whichever of these predictions turns out to be correct, the translation of the science into clinical practice for reproductive purposes is likely to be a protracted process so IVG is not going to be available imminently in clinical settings.
2. IVG could end gamete shortages for treatment and research IVG could transform the treatment of those with certain types of infertility. A major issue in reproductive medicine is the scarcity of viable donor eggs and sperm. If it becomes possible to generate human IVD gametes from non-reproductive bodily cells at scale, viable eggs and sperm could become more readily available for treatment and research.
IVG could transform the treatment of those with certain types of infertility. A major issue in reproductive medicine is the scarcity of viable donor eggs and sperm. If it becomes possible to generate human IVD gametes from non-reproductive bodily cells at scale, viable eggs and sperm could become more readily available for treatment and research.
If gamete scarcity were to end as a result of the introduction of IVG, this could have a number of impacts. Firstly, it could result in reduced demand for donor gametes as these won’t be needed in most cases if they can be generated from the patient’s own body cells. Second, it could radically change the IVF treatment cycle. Currently, if a female patient wants to reproduce using their own eggs, they have to go through an intrusive egg harvesting procedure which comes with associated health risks (e.g. pelvic infection, ovarian hyperstimulation syndrome). If those eggs can be made in the laboratory instead, then the egg harvesting phase of IVF treatment could be eliminated. As a consequence, it would be possible to have more rounds of IVF potentially resulting in higher overall success rates. Finally, it could lead to more gametes being available for research purposes enabling scientists to understand more about why some types of infertility occur and support the development of new drugs, for example non-hormonal contraceptives.
3. IVG could enable new types of biological family
 As well as transforming reproductive medicine, IVG could also increase reproductive choice for those who have previously been unable to have genetically related children.
As well as transforming reproductive medicine, IVG could also increase reproductive choice for those who have previously been unable to have genetically related children.
If eggs and sperm can be generated from both male and female body cells, there is the potential for same-sex couples to have children that are genetically-related to both partners. For example, in a same-sex female couple one partner would provide the egg and the other partner would provide the sperm negating the current need for third-party (sperm donor) involvement. For male same-sex couples the same IVG process would apply; however, they would still need to use a surrogate.
Much has been made of the potential of IVG to enable solo genetic parenting where one person provides both egg and sperm. However, because the resulting embryo will have double copies of all of the genes, the risks of autosomal recessive disease are so high that it is likely that any embryos conceived in this way would either not survive or would have a high risk of at least one serious genetic disease. This is not to say that solo genetic parenting will never be possible, but for the embryo to be healthy it would require an intervention such as pre-implantation genetic testing or carrier screening (which are currently available in some countries although do not offer coverage of every gene) or genetic editing of the embryo which is currently prohibited.
IVG could also lead to more families with older (especially female) genetic parents and level the playing field in terms of the biological age limits on reproduction, which disproportionately disadvantage women. During the menopause, which in most women occurs between the ages of 45 and 55, the ovaries cease producing eggs so unassisted biological reproduction is no longer possible; whereas most men go on creating sperm into old age and can become biological fathers much later in life. IVG could enable women to continue to reproduce beyond the menopause and also offer an alternative to social egg freezing. If older women could create gametes from their own non-reproductive cells, there would be no need for them to freeze eggs when they are younger.
Other Posts
- The Future of Artificial Placenta Technology: Should we Proceed with Caution?
- Spotlight on CRISPR-Cas9
- Exhibition Review: Genetic Automata
- A Look Back at 2023
- Explainer: Human Stem Cell Based Embryo Models
- Is it time to revisit the 14-day rule?
- Gifting the Womb: The UK’s First Uterus Transplantation
- Discipline Hopping: UK Law and Emerging Reproductive Technologies
- Reflections: A Look Back at the First Year of the Project
- Book Review – Eve: The Disobedient Future of Birth
- Ectogenesis: A Retrospective
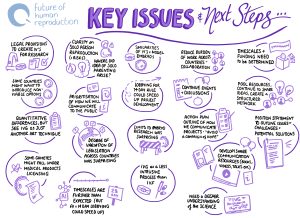
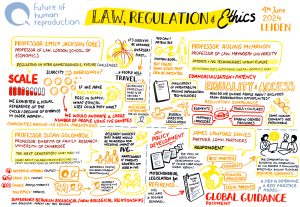
4. Legislative barriers could slow down the development and introduction of IVG
 Legislation relating to human reproduction varies widely across jurisdictions and there are a number of current barriers to the development and introduction of IVG.
Legislation relating to human reproduction varies widely across jurisdictions and there are a number of current barriers to the development and introduction of IVG.
To understand how human germ cells originate, and to confirm the similarity of laboratory-made gametes to those produced in the human body, it is necessary to research the early stages of human embryo development. However, there are various restrictions on embryo research that are prohibiting scientific progress with IVG.
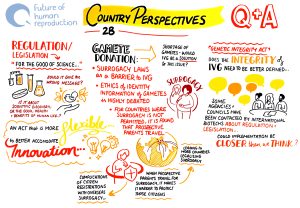
In countries such as Austria, Germany, Italy and Turkey, there is an outright ban on embryo research. In Canada, Japan, the Netherlands and Spain, while embryo research is allowed, it is illegal to create an embryo for this purpose – only donated IVF embryos can be used, leading to a scarcity of high-quality embryos for research.
In many jurisdictions where embryo research is permitted, a 14-day limit on the cultivation of the embryos applies. It is around the 14-day mark that gastrulation starts. Gastrulation is a critical stage in human embryo development where the primary cell layers are formed and the process of developing organs and tissues begins, including germ cell specification. As a result of the 14-day limit, scientists in many countries cannot investigate germline development. There have been calls to extend the limit to 28-days – the Health Council of the Netherlands, for example, recently made a recommendation to this effect – but so far there haven’t been any changes to the law in those countries that have adopted the 14-day rule.
In response, scientists have been developing Stem Cell Based Embryo Models (SCBEMs), embryo-like structures that mimic the processes that occur in early human embryo development, to investigate, amongst other things, germline development. However, these models raise their own ethical and regulatory issues, which led the International Society for Stem Cell Research (ISSCR) to publish a statement on research with these entities in 2023.
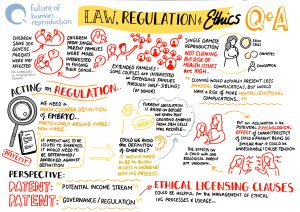
In some countries, IVG is prohibited for use in human reproduction under current legislation. This is the case in the UK because, while IVG is not specifically mentioned, Section 3 of the Human Fertilisation and Embryology Act (as amended in 2008) has definitions of ‘permitted’ sperm, eggs and embryos that exclude IVD gametes and embryos created from those gametes. Only ‘permitted’ embryos and gametes can be lawfully placed in a woman. In other countries, IVG is not accounted for at all in current legislation.
There is also a question of whether the definitions of legal parenthood, in different jurisdictions, will be able to accommodate the new forms of biological family that IVG could enable, and whether bans on surrogacy (for example, in Italy, France, Germany and Norway) will need to be reviewed to ensure equitable access to the benefits of IVG. These bans, for example, could disproportionately disadvantage male same-sex couples who will need to use a surrogate in order to make use of IVG to create their own genetically-related child.
There is, then, an urgent need to review current frameworks to ensure that they are capable of dealing with anticipated developments in IVG technology. Failure to do so could have negative effects such as an increase in patients travelling for treatment to countries where reproductive services are underregulated.
5. IVG raises ethical issues
 As with the introduction of any new reproductive biotechnology, IVG raises a number of ethical challenges.
As with the introduction of any new reproductive biotechnology, IVG raises a number of ethical challenges.
One of the core issues is how to determine when IVG is sufficiently safe for use in the human reproductive context, who should decide this, and how they should decide. Even with extensive pre-clinical and clinical trials, no new technology is ever going to be completely risk-free and so a decision needs to be made about what constitutes an acceptable level of risk and how this acceptability is determined. For example, is it determined comparatively (is the risk higher than in natural reproduction or other alternative ways of becoming a parent?); by delineating an acceptable risk threshold (if the risk is not higher than X% then it is deemed safe); or by proportionality (do the risks/disadvantages outweigh the benefits?)? And what if future users of IVG are prepared to accept a higher level of risk so that they can have a genetically-related child?
There are also risks beyond practical questions of clinical safety, such as the potential for IVG to be misused. Specific concerns include the possibility of using IVG to bring about gamete theft, unwitting parenthood, or inappropriate uses of selective reproduction or genetic modification. The latter is a particularly live issue because, while a combination of genome editing and IVG could potentially avoid some of the off-target effects that are created during cell transformation, it could also enable the extensive genome editing of germ cells for non-medical reasons.
If IVG is, at some point, deemed safe for clinical use, it could have both a medical benefit (enabling those with certain types of infertility to have a genetically-related child); and a social benefit (democratising reproduction to enable groups that were previously excluded from having a genetically-related child to do so). However, this raises a series of further questions. Which uses of the technology should be permitted and why? Who should have access to treatment and how will this be determined? How will it be funded? There are justifiable concerns that access to IVG will be inequitable either because the costs will be prohibitive or because certain groups will be purposely excluded from accessing the technology.
The status of the IVG-created embryo and the welfare of children born using IVG also merits consideration. Are there any moral differences between an IVG embryo, an IVF embryo and an embryo created through biological reproduction and, if so, what are they? Is a child born using IVG any different from a child born via IVF or unassisted conception, and do they have a right to know that they were conceived using IVG and about the origins of the IVD gametes used?
Finally, there is the issue of whether it is right to privilege genetic-relatedness over other types of parent-child relationship and what this could mean for those who choose alternative routes to parenthood, such as donor gametes or adoption. Might the introduction of IVG call into doubt the choices of those who decide that they do not prefer genetically-related offspring?
Tackling the complex ethical challenges raised by IVG requires a plurality of perspectives and it is important that there are opportunities for policy-makers, regulators, academics, scientists and potential users of the technology to be involved in the debate. IVG should not be used for human reproduction until these pressing issues have been properly considered.
6. Engaging with the public about IVG is vital
 There is currently a lack of public awareness about IVG research, although some groups have been consulted as part of smaller public engagement initiatives in the Netherlands, UK and US. Anecdotal evidence from these suggests that there is sympathy and support for IVG in some contexts (e.g. as an alternative for infertile couples) but not in others (e.g. for post-menopausal women); and that it is seen as a potentially positive alternative to existing interventions such as egg retrieval and gamete donation.
There is currently a lack of public awareness about IVG research, although some groups have been consulted as part of smaller public engagement initiatives in the Netherlands, UK and US. Anecdotal evidence from these suggests that there is sympathy and support for IVG in some contexts (e.g. as an alternative for infertile couples) but not in others (e.g. for post-menopausal women); and that it is seen as a potentially positive alternative to existing interventions such as egg retrieval and gamete donation.
More work needs to be done to inform people about IVG because if it does become available for use in human reproduction, it could have far-reaching social implications. It is vitally important to engage with the public, to ensure that they are heard, can learn enough so that they can contribute in a meaningful way, and are actively involved in decision-making processes. This is key if there is going to be public trust in the science of IVG and in the regulatory frameworks that will oversee its introduction.
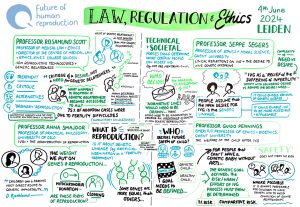
This blog is based on discussions that took place at the IVG Ethics and Policy Symposium on 3rd and 4th June 2024 in Leiden, the Netherlands. The Future of Human Reproduction team would like to thank the panel chairs, speakers and participants for their insightful and thought-provoking contributions.
The Symposium was funded by the UKRI QR Policy Support Fund at Lancaster University.
The full Symposium programme can be found here: IVG Symposium Schedule.