Spotlight on CRISPR-Cas9
By Dr Zoe Bolton, published 9th February 2024
To mark International Day of Women and Girls in Science we’re recognising the groundbreaking work of Profs Emmanuelle Charpentier and Jennifer Doudna, who became the first all-female team to win a Nobel Prize in Chemistry for the development of the CRISPR-Cas9 gene editing tool. This blog, celebrating their achievement, traces the discovery of CRISPR-Cas9; explores the current applications and risks of the technology; and considers how it might be used in reproductive contexts in the future and the associated ethical issues that this raises.
What is CRISPR-Cas9?
CRISPR-Cas9 is a form of genome editing, which enables scientists to perform microsurgery on the DNA of living organisms such as animals, plants and insects. It is effectively a molecular Swiss army knife, with multiple functionality, that can delete, repair or replace specific sections of DNA.
 CRISPRs (Clustered Regularly Interspaced Short Palindromic Repeats) and CRISPR associated (Cas) enzymes (nucleases that breakdown threatening non-host DNA), were first identified in prokaryotes. These are microscopic single cell organisms, such as bacteria, that do not have a nucleus or other subcellular structures. Prokaryotes are susceptible to viruses (bacteriophages) which are specific to them; these can be lethal or result in other adverse genetic changes.
CRISPRs (Clustered Regularly Interspaced Short Palindromic Repeats) and CRISPR associated (Cas) enzymes (nucleases that breakdown threatening non-host DNA), were first identified in prokaryotes. These are microscopic single cell organisms, such as bacteria, that do not have a nucleus or other subcellular structures. Prokaryotes are susceptible to viruses (bacteriophages) which are specific to them; these can be lethal or result in other adverse genetic changes.
Scientists discovered that prokaryotes develop a genetic defence mechanism for protection against repeated viral attacks. They do this by inserting spacers, derived from invading genetic elements, into clusters of short, repeating sequences of DNA in a genomic area known as the CRISPR array. These spacers serve as a genetic memory of previous viral infections so that the next time the prokaryote is infected with the same virus it can direct a Cas enzyme to the site of the invading DNA and destroy it.
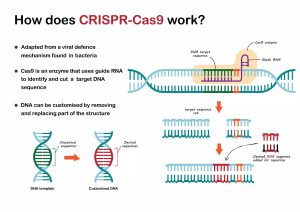 Prokaryotes, then, have a natural gene editing system for immunisation, and Charpentier and Doudna transformed this system into a revolutionary genome editing method capable of recognising, cleaving and repairing any genomic sequence. In 2012 they published the paper that would eventually lead to the Nobel Prize in Chemistry. In this paper, they describe engineering two molecules CRISPR-associated RNA (crRNA) and trans-activating CRISPR RNA (tracrRNA) – which are responsible for directing the Cas9 protein so it can identify and cleave invading DNA – into a single guide RNA (sgRNA). This simplified the CRISPR system because the sgRNA is capable of being reprogrammed so that Cas9 can target any DNA sequence and initiate a site-specific, double-stranded break. As Charpentier and Doudna note in their paper, the method offers ‘considerable potential for gene targeting and genome editing applications’.
Prokaryotes, then, have a natural gene editing system for immunisation, and Charpentier and Doudna transformed this system into a revolutionary genome editing method capable of recognising, cleaving and repairing any genomic sequence. In 2012 they published the paper that would eventually lead to the Nobel Prize in Chemistry. In this paper, they describe engineering two molecules CRISPR-associated RNA (crRNA) and trans-activating CRISPR RNA (tracrRNA) – which are responsible for directing the Cas9 protein so it can identify and cleave invading DNA – into a single guide RNA (sgRNA). This simplified the CRISPR system because the sgRNA is capable of being reprogrammed so that Cas9 can target any DNA sequence and initiate a site-specific, double-stranded break. As Charpentier and Doudna note in their paper, the method offers ‘considerable potential for gene targeting and genome editing applications’.
Applications of CRISPR-Cas9
Since 2012 that ‘considerable potential’ has been realised and CRISPR-Cas9 has been adopted with increasing precision in a wide range of contexts. It has been used to create gene knock-ins (the targeted insertion of a DNA sequence at a specific site in the genome); gene knock-outs (the removal or inactivation of a gene or genes from an organism); and point mutations (the alteration of a single base pair in the DNA sequence).

In plant science, CRISPR-Cas9 has improved crop nutrition and tolerance to climate shocks, and increased disease resistance. It has also been used in animal breeding to increase productivity and disease resistance; to reduce the chances of rejection in cross-species organ transfers; and in insect populations as a potential tool to control the transmission of vector-borne diseases such as malaria. In medical science, CRISPR-Cas9 is being used in trials for the treatment of diseases, for example certain types of cancer, and in attempts to develop gene therapies for conditions including cystic fibrosis and Duchenne muscular dystrophy.
In November 2023, the UK Medicines and Healthcare products Regulatory Agency (MHRA) authorised the world’s first therapeutic use of CRISPR-Cas9 gene editing to treat the genetic blood disorders sickle-cell disease and beta thalassemia. It is important to note that these therapeutic uses of CRISPR-Cas9 are not heritable because, although the DNA of living humans is altered, the reproductive cells (eggs and sperm) remain unchanged meaning that the alterations to the DNA cannot be passed on to future generations.
Risks of CRISPR-Cas9
As the many applications of CRISPR-Cas9 show, Charpentier and Doudna’s remarkable innovation has transformed the field of genome editing. It has provided a simple, efficient and cost-effective tool that can be used to edit the DNA of any organism. However, as with any new biotechnology, there are risks associated with CRISPR-Cas9. The most significant of these risks is off-target effects including off-target cutting, mutations and cleavage. Research is already underway to minimise the impact of such effects but they remain an ongoing concern as they can destabilise the genome, disrupt the function of otherwise healthy genes and introduce potentially harmful genetic mutations into the DNA of the organism.
 In addition to the risks of off-target effects, the ethical challenges raised by CRISPR-Cas9 are well documented. For example, where CRISPR has been used in food systems, there are concerns about the safety of genetically modified crops and about the welfare of the animals that are being intensively farmed to increase production. Its use in ecological contexts, such as in the modification of insects, raises issues relating to the disruption of fragile ecosystems, species eradication and the possibility of other significant, unintended environmental consequences. Finally, there are concerns about the potential for CRISPR to be used for nefarious purposes such as bioterrorism.
In addition to the risks of off-target effects, the ethical challenges raised by CRISPR-Cas9 are well documented. For example, where CRISPR has been used in food systems, there are concerns about the safety of genetically modified crops and about the welfare of the animals that are being intensively farmed to increase production. Its use in ecological contexts, such as in the modification of insects, raises issues relating to the disruption of fragile ecosystems, species eradication and the possibility of other significant, unintended environmental consequences. Finally, there are concerns about the potential for CRISPR to be used for nefarious purposes such as bioterrorism.
CRISPR-Cas9 and human reproduction
A significant focus of the ethical debate about genome editing has been on its potential application in the human reproductive context. There was widespread controversy in 2018 when a Chinese biophysicist, Dr He Jiankui, shocked the global scientific community by announcing the birth of non-identical twin girls created from embryos that had been genetically edited.
Using CRISPR techniques, He Jiankui claimed to have modified the genes of the twins to make them immune to HIV, the virus that causes AIDS. He Jiankui’s work was widely condemned as irresponsible, leading to a prominent group of researchers, including Charpentier, to call for a global moratorium on the editing of DNA in human sperm, eggs or embryos to make genetically modified children. He Jiankui was subsequently sentenced to three years in prison after being found guilty of conducting illegal medical practices.
The situation that arose in China is highly unlikely to happen in the UK because of strict regulation. Gene editing for reproductive purposes is illegal and, while gene editing research on human embryos can be permitted under license from the Human Fertilisation and Embryology Authority (HFEA), the 14-day rule currently prevents the use of embryos for research beyond that time limit. The HFEA first approved the use of CRISPR-Cas9 in human embryo research in 2016.
 Given how stringent the UK law is in this area, those with heritable genetic conditions who want to have genetically related children, without transmitting those conditions, have to rely on other options. One alternative, which results in a partially genetically related child, is ‘third party reproduction’ whereby an egg or sperm (gamete) donor is used in in vitro fertilisation (IVF) in place of the gamete from the affected individual in the couple. This is obviously not a viable choice in a situation where both parents have genetic abnormalities; in which case two donors would be required and there would then be no genetic link between the parents and the child.
Given how stringent the UK law is in this area, those with heritable genetic conditions who want to have genetically related children, without transmitting those conditions, have to rely on other options. One alternative, which results in a partially genetically related child, is ‘third party reproduction’ whereby an egg or sperm (gamete) donor is used in in vitro fertilisation (IVF) in place of the gamete from the affected individual in the couple. This is obviously not a viable choice in a situation where both parents have genetic abnormalities; in which case two donors would be required and there would then be no genetic link between the parents and the child.
Another option is preimplantation genetic testing (PGT). This is a technique used to identify genetic abnormalities in embryos created through IVF so that the embryos which do not contain the abnormality can be implanted into the womb. However, there are some cases where PGT is not effective because the genetic makeup of the parents means that it is not possible, or highly unlikely, that an unaffected embryo can be produced for selection. This is the case for dominant conditions, such as Huntington’s disease, where one parent carries two copies of the gene; and for recessive conditions, such as cystic fibrosis, where both parents carry two copies of the gene. In these exceptional cases, CRISPR-Cas9 genome editing could offer a solution, if there is a change to the law, but it raises a number of ethical issues.
 First and foremost, are concerns about the implications of introducing heritable changes into the human gene pool when the impact of such changes on future generations cannot be known. As previously mentioned, there is a risk of off-target effects from CRISPR-Cas9. There are also still significant unknowns about early human embryo development because of the scarcity of high-quality embryos for research and the 14-day rule. In combination, this means that it is currently difficult to predict with accuracy what the consequences of genome editing on a human embryo might be both for the development of the embryo itself and for the potential child that might result from that embryo (and consequently their future children).
First and foremost, are concerns about the implications of introducing heritable changes into the human gene pool when the impact of such changes on future generations cannot be known. As previously mentioned, there is a risk of off-target effects from CRISPR-Cas9. There are also still significant unknowns about early human embryo development because of the scarcity of high-quality embryos for research and the 14-day rule. In combination, this means that it is currently difficult to predict with accuracy what the consequences of genome editing on a human embryo might be both for the development of the embryo itself and for the potential child that might result from that embryo (and consequently their future children).
There is, then, a pressing safety issue that needs to be addressed before CRISPR-Cas9 can be sanctioned for reproductive purposes. More research is needed to ensure that the benefits outweigh the risks, particularly when non-heritable therapeutic gene editing applications might become available for some genetic conditions and offer a less risky alternative.
In the event that CRISPR-Cas9 is at some point deemed safe to use on human embryos for the prevention of heritable conditions, this could have an impact on perceptions of disability. If certain types of disability can be almost eradicated through genetic editing this could lead to the stigmatising of those living with some conditions and generate a negative view about disability: the idea that some lives are ‘worth more’ than others. It could also result in a less diverse population where some desirable characteristics are lost in the attempt to eliminate other genetic abnormalities.
Genome editing could be viewed, by some, as a ‘eugenic’ practice, whereby perceived ‘undesirable’ traits are being edited out of the population; and also as a slippery slope leading to the selection of other genetic characteristics for non-medical reasons. For example, parents who can afford to pay being able to choose the desired physical attributes of a child (sometimes called ‘liberal eugenics’). Related to this, are issues of access and funding. If access is unequally distributed, because of cost, then it could drive social inequality and exacerbate social divisions. This may especially be the case if a point is reached where genome editing becomes ‘normal’ in the reproductive context, calling into doubt the choices of those who opt to conceive without using the technology.
 There is also the question of whether genetic relatedness should be privileged over other types of parent-child relationship: for example, adoption or creating children using donor eggs and sperm. However, this consideration must be balanced with respecting an individual’s autonomy and their desire for genetic parenthood unless there are unacceptable risks or harmful consequences of doing so. If genome editing does become safe and effective in the reproductive context, then it may become the more ethically acceptable choice for an individual or couple with a genetic condition because it will increase the chances of healthy offspring and it does not require someone else to have an invasive procedure as is the case with egg donation.
There is also the question of whether genetic relatedness should be privileged over other types of parent-child relationship: for example, adoption or creating children using donor eggs and sperm. However, this consideration must be balanced with respecting an individual’s autonomy and their desire for genetic parenthood unless there are unacceptable risks or harmful consequences of doing so. If genome editing does become safe and effective in the reproductive context, then it may become the more ethically acceptable choice for an individual or couple with a genetic condition because it will increase the chances of healthy offspring and it does not require someone else to have an invasive procedure as is the case with egg donation.
Many of the ethical issues raised by the application of CRISPR-Cas9 in the human reproductive context are the same as those that arise with other reproductive technologies and are the inevitable result of scientific progress. While it is crucial that these issues are addressed, preferably through an open, transparent and meaningful dialogue involving researchers and the public, it is also important to recognise the contribution of those driving innovation with the aim of improving people’s lives. Charpentier and Doudna’s methodological breakthrough, and the developments that it has enabled and continues to enable, has rightfully earned them a place in scientific history.
Other Posts
- “The genetics bomb could be a disaster”: Author Simon Mawer on Mendel’s Dwarf
- Exhibition Review: Genetic Automata
- A Look Back at 2023
- Explainer: Human Stem Cell Based Embryo Models
- Is it time to revisit the 14-day rule?
- Gifting the Womb: The UK’s First Uterus Transplantation
- Discipline Hopping: UK Law and Emerging Reproductive Technologies
- Reflections: A Look Back at the First Year of the Project
- Book Review – Eve: The Disobedient Future of Birth
- Ectogenesis: A Retrospective
