Prof Munir is a virologist with research experience in virus pathobiology, viral antagonism of immune responses, and host factors that limit virus replication. Research in Munir’s laboratory focuses on understanding molecular mechanisms of inter-species pathogenesis of viruses (i.e., zoonotic viruses). Specifically, using influenza viruses and coronaviruses (e.g., SARS-CoV-2, the causative agent of CoVID-19), his research aims to explore host and viral RNA biology (sensing – interferon responses, epigenetics, and gene regulation) and define structural and functional differences in human and animals (e.g., birds and bats), which determine the transmission dynamics of animal viruses to human.
Munir’s laboratory is funded by the BBSRC, British Council, Newton Fund, and Industry, and is equipped with CL3 facilities, holding avian viruses bank, chicken eggs facilities, and expanding to establish vaccine vectors bank.
Specifically, research in The Munir’s Lab focuses on novel vaccine development, innovative diagnostic methods, and understanding host-pathogen interactions at genetics, epigenetics, and molecular levels.
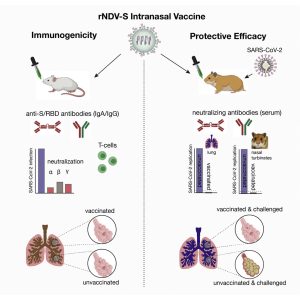 Novel Vaccines Development: We are at the forefront of developing vectored nasal vaccine against COVID-19. Our recent study in mice and hamsters has demonstrated immunogenicity, safety, and protective efficacy against SARS-CoV-2. Intranasal administration of recombinant vaccine expressing spike (S) protein of SARS-CoV-2 to mice induced high levels of SARS-CoV-2-specific neutralizing immunoglobulin A (IgA) and IgG2a antibodies and T-cell-mediated immunity. Hamsters immunized with two doses of the vaccine showed complete protection from a lung infection, inflammation, and pathological lesions following the SARS-CoV-2 challenge. Importantly, the administration of two doses of intranasal vaccine significantly reduced the SARS-CoV-2 shedding in nasal turbinate and lungs in hamsters. Collectively, intranasal vaccination has the potential to control infection at the site of inoculation, which should prevent both clinical disease and virus transmission to halt the spread of the COVID-19 pandemic. Read More
Novel Vaccines Development: We are at the forefront of developing vectored nasal vaccine against COVID-19. Our recent study in mice and hamsters has demonstrated immunogenicity, safety, and protective efficacy against SARS-CoV-2. Intranasal administration of recombinant vaccine expressing spike (S) protein of SARS-CoV-2 to mice induced high levels of SARS-CoV-2-specific neutralizing immunoglobulin A (IgA) and IgG2a antibodies and T-cell-mediated immunity. Hamsters immunized with two doses of the vaccine showed complete protection from a lung infection, inflammation, and pathological lesions following the SARS-CoV-2 challenge. Importantly, the administration of two doses of intranasal vaccine significantly reduced the SARS-CoV-2 shedding in nasal turbinate and lungs in hamsters. Collectively, intranasal vaccination has the potential to control infection at the site of inoculation, which should prevent both clinical disease and virus transmission to halt the spread of the COVID-19 pandemic. Read More
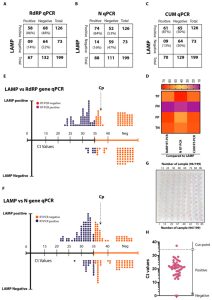 Innovative Diagnostic Methods: We have developed a de novo, high-resolution, and comparative genomics-guided reverse-transcribed loop-mediated isothermal amplification (LAMP) assay. To further enhance the assay performance and to remove any subjectivity associated with operator interpretation of results, we engineered a novel hand-held smart diagnostic device. The robust diagnostic device was further furnished with automated image acquisition and processing algorithms and the collated data was processed through artificial intelligence (AI) pipelines to further reduce the assay run time and the subjectivity of the colorimetric LAMP detection. This advanced AI algorithm-implemented LAMP (ai-LAMP) assay, targeting the RNA-dependent RNA polymerase gene, showed high analytical sensitivity and specificity for SARS-CoV-2. A total of ~200 coronavirus disease (CoVID-19)-suspected NHS patient samples were tested using the platform and it was shown to be reliable, highly specific, and significantly more sensitive than the current gold standard qRT-PCR. Therefore, this system could provide an efficient and cost-effective platform to detect SARS-CoV-2 in resource-limited laboratories.
Innovative Diagnostic Methods: We have developed a de novo, high-resolution, and comparative genomics-guided reverse-transcribed loop-mediated isothermal amplification (LAMP) assay. To further enhance the assay performance and to remove any subjectivity associated with operator interpretation of results, we engineered a novel hand-held smart diagnostic device. The robust diagnostic device was further furnished with automated image acquisition and processing algorithms and the collated data was processed through artificial intelligence (AI) pipelines to further reduce the assay run time and the subjectivity of the colorimetric LAMP detection. This advanced AI algorithm-implemented LAMP (ai-LAMP) assay, targeting the RNA-dependent RNA polymerase gene, showed high analytical sensitivity and specificity for SARS-CoV-2. A total of ~200 coronavirus disease (CoVID-19)-suspected NHS patient samples were tested using the platform and it was shown to be reliable, highly specific, and significantly more sensitive than the current gold standard qRT-PCR. Therefore, this system could provide an efficient and cost-effective platform to detect SARS-CoV-2 in resource-limited laboratories.
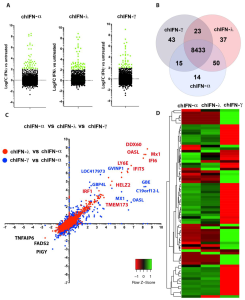
Host-pathogen Interactions: At Prof Munir’s Lab, host-pathogen interactions are being studied at epitranscriptomic, genetics, and molecular levels ranging from sensing – interferon responses, epigenetics, and gene regulation and define structural and functional differences in human and animals (e.g. birds and bats), which determine the transmission dynamics of animal viruses to human. Key scientific contributions include the identification of novel interferon (e.g., IFN-kappa), the introduction of a novel concept and terminology “reverse spillover” amongst others
